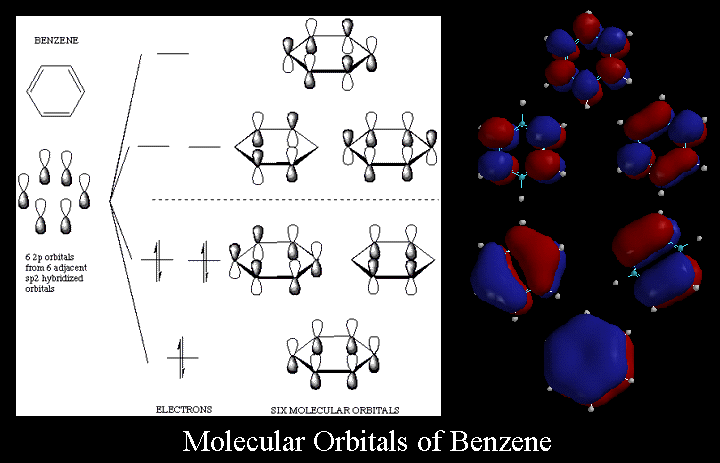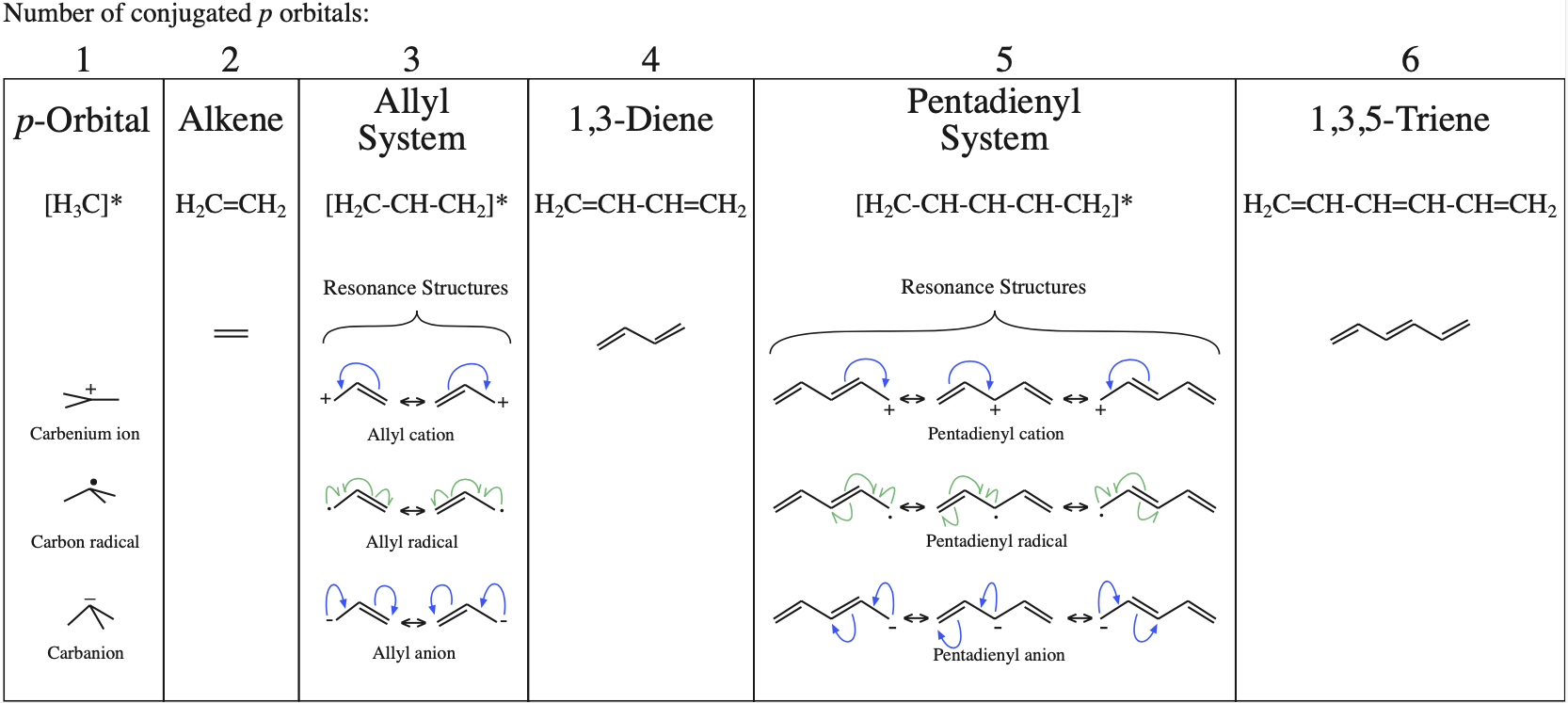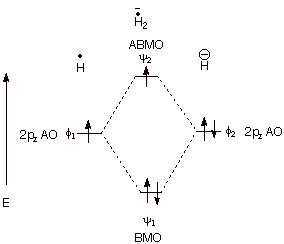
1. Strong Covalent Bonds. Consider the pi bond of ethene in simple molecular orbital terms (The qualitative results would be the same for any pi or sigma bond.

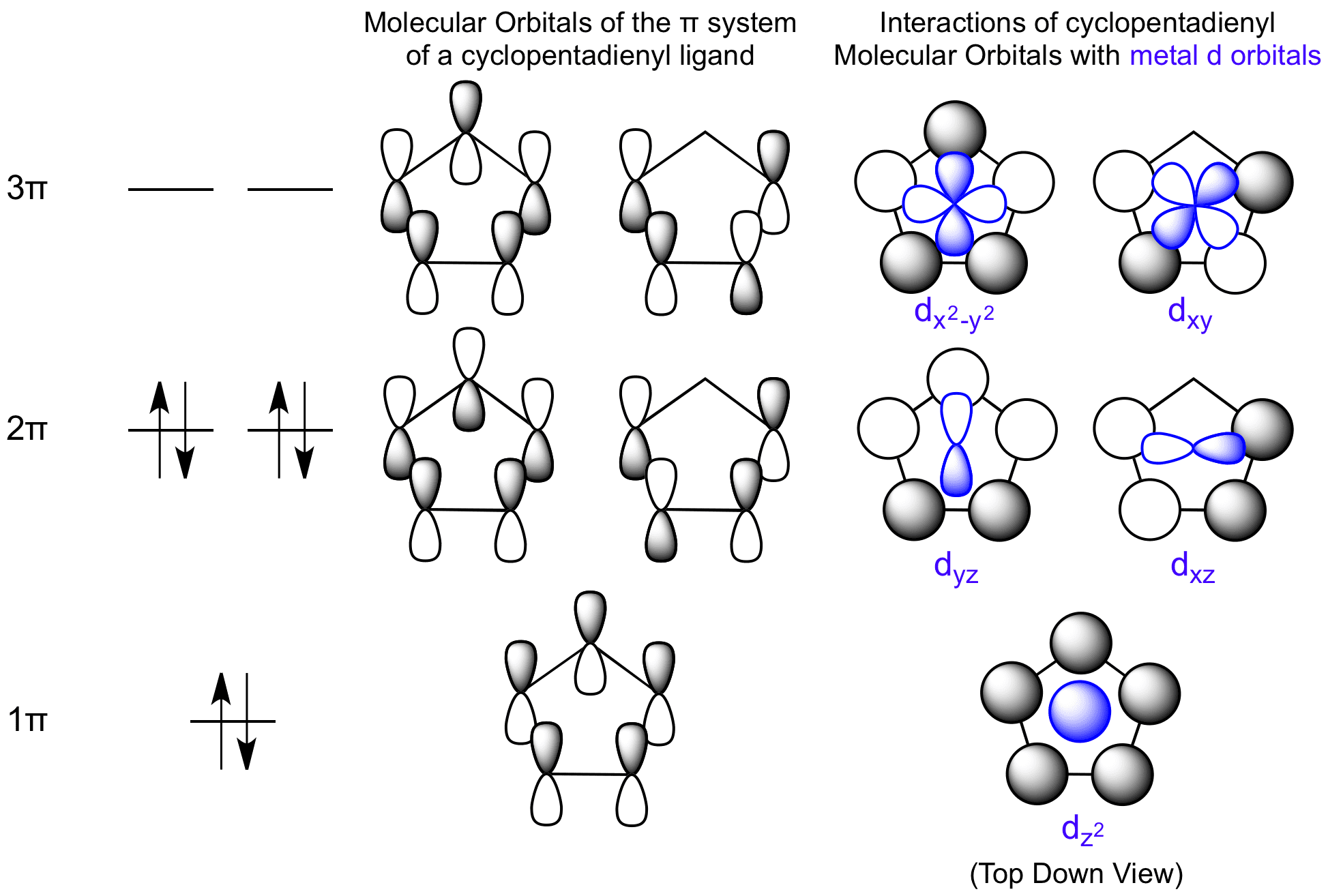
orbitals of cyclopentadienyl radical. Because of the fivefold symmetry,... | Download Scientific Diagram

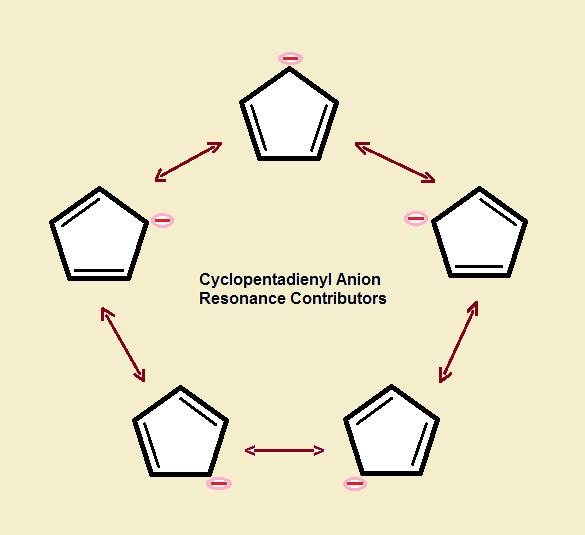
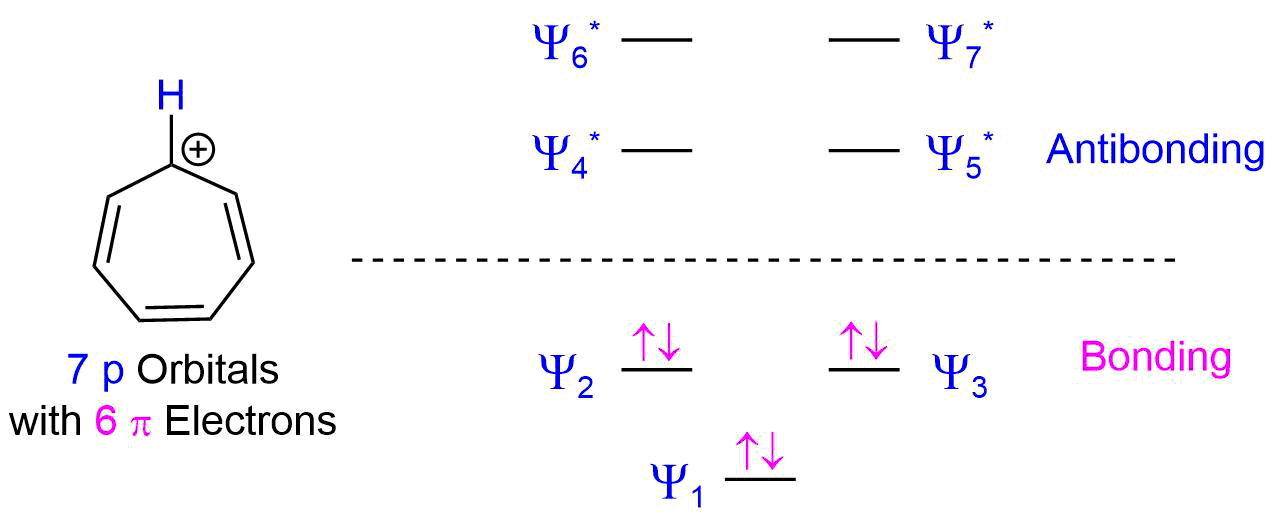
Describe the electron distribution in the Mos of the cyclopentadienyl anion. Strategy: Use the polygon-and-circle method for deriving the relative energies of the pi Mos
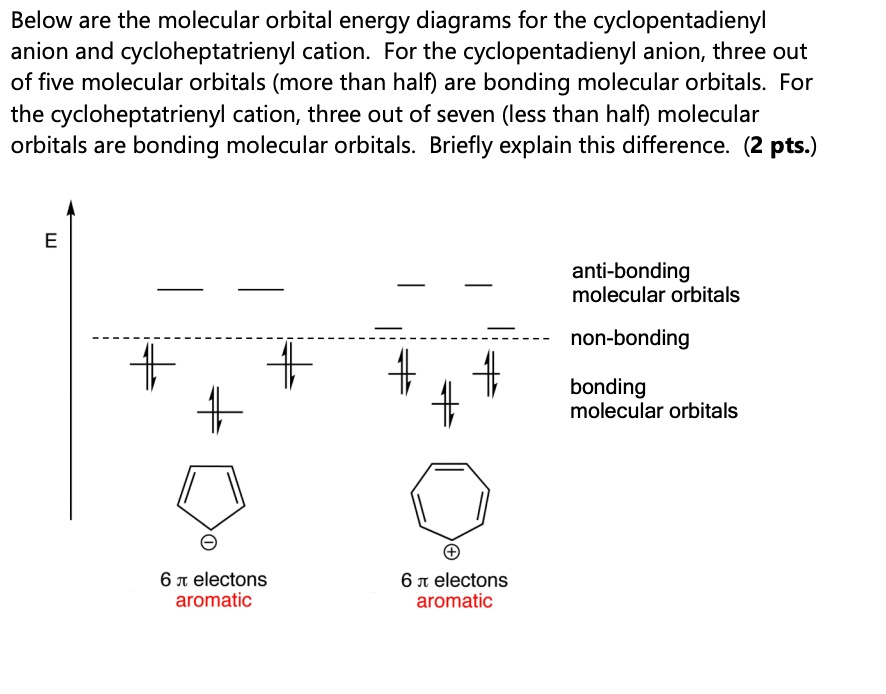
SOLVED: Below are the molecular orbital energy diagrams for the cyclopentadienyl anion and cycloheptatrienyl cation. For the cyclopentadienyl anion, three out of five molecular orbitals (more than half) are bonding molecular orbitals

organic chemistry - Cyclopentadienyl radical geometry and MO considerations - Chemistry Stack Exchange

The relative energy levels of the five pi molecular orbitals of the cyclopentadienyl system are similar to those in benzene. That is, there is a single lowestenergy MO, above which the orbitals
![PDF] Cyclopentadienyl System: Solving the Secular Determinant, π Energy, Delocalization Energy, Wave Functions, Electron Density and Charge Density | Semantic Scholar PDF] Cyclopentadienyl System: Solving the Secular Determinant, π Energy, Delocalization Energy, Wave Functions, Electron Density and Charge Density | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4620a9b0ed458b645be7ab1e2807b0b2e56bcd1e/2-Figure5-1.png)
PDF] Cyclopentadienyl System: Solving the Secular Determinant, π Energy, Delocalization Energy, Wave Functions, Electron Density and Charge Density | Semantic Scholar

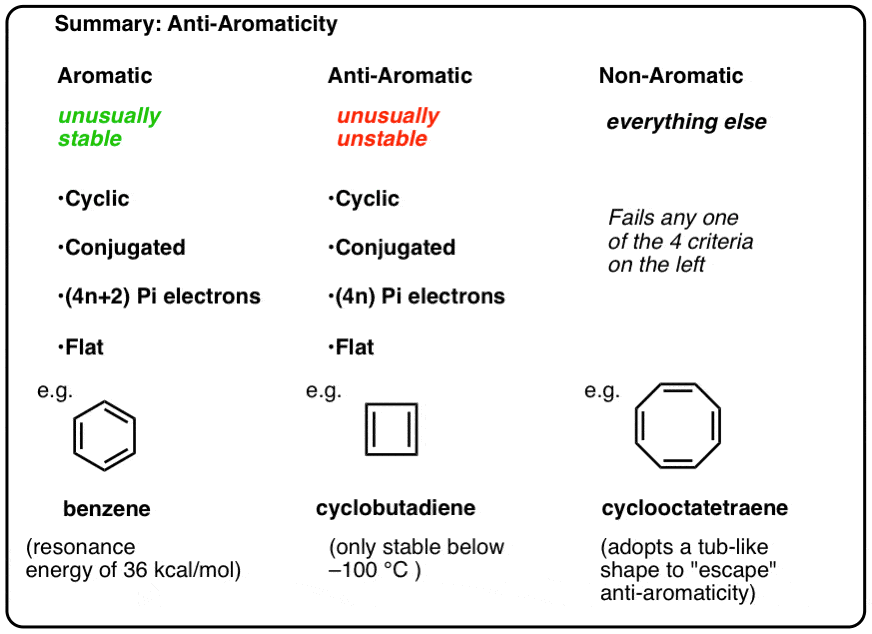
Use the inscribed polygon method to show why the cyclopentadienyl cation and radical are not aromatic. | Homework.Study.com