Standard reduction potential of different species in aqueous solution... | Download Scientific Diagram

Table 4 from Nickel(II) complexes with tetra- and pentadentate aminopyridine ligands: synthesis, structure, electrochemistry, and reduction to nickel(I) species. | Semantic Scholar
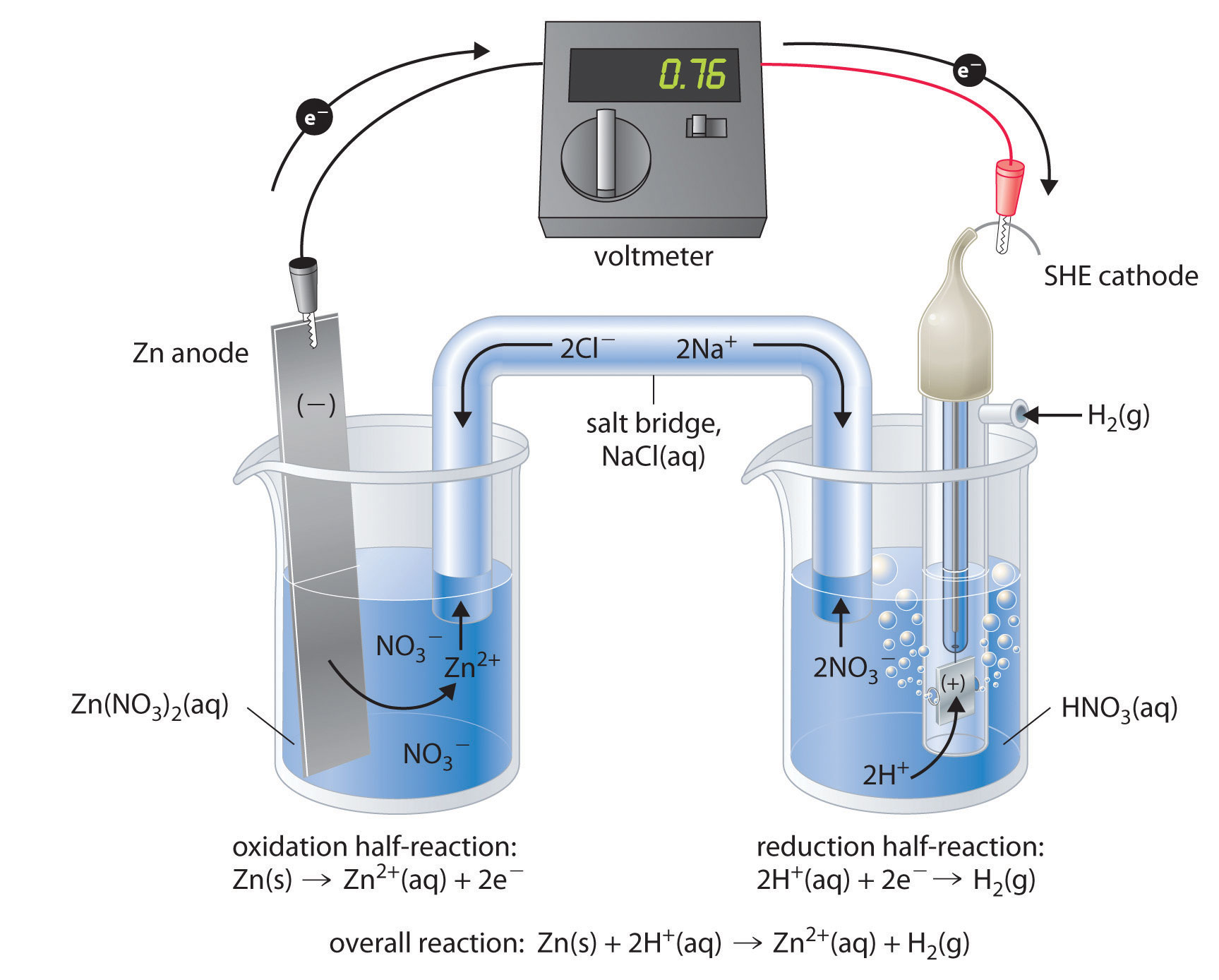
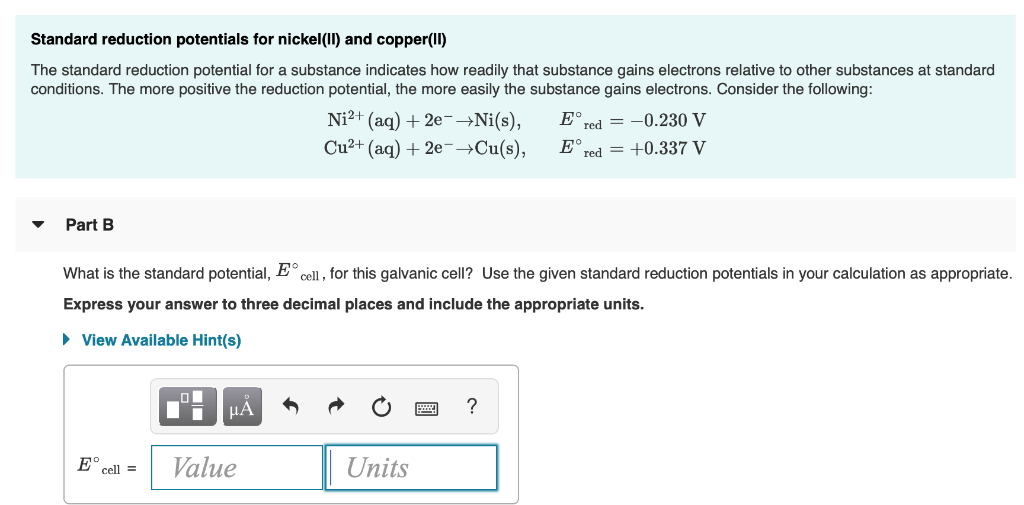
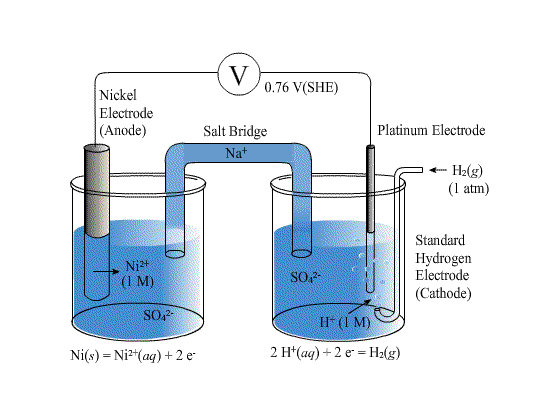
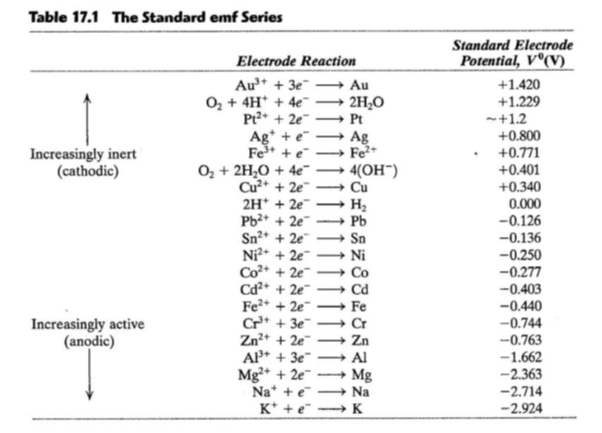
The standard reduction potentials for Zn^2+ / Zn, Ni^2+ /Ni and Fe^2+ / Fe are - 0.76, -0.23 and - 0.44V, respectively. - Sarthaks eConnect | Largest Online Education Community

Table 1 from Co-electrodeposited Mesoporous PtM ( M = Co , Ni , Cu ) as an Active Catalyst for Oxygen Reduction Reaction in a PEMFC | Semantic Scholar

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential ... - YouTube
![Expert Answer] calculate standard electrode potential of Ni/Ni2+,if cell potential of the - Brainly.in Expert Answer] calculate standard electrode potential of Ni/Ni2+,if cell potential of the - Brainly.in](https://hi-static.z-dn.net/files/d51/b7359f4685f931af8cd83cf0636289d3.jpg)
Expert Answer] calculate standard electrode potential of Ni/Ni2+,if cell potential of the - Brainly.in

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential potential of the cell, Ni//N^(2+)(0.01 M)//Cu is 0.59" V ". "Given" E(Cu^(2+)//Cu)^(@)=+0.34 " V "

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa

Calculate the standard electrode potential of Ni2+/Ni electrode if |Class 12 CHEMISTRY | Doubtnut - YouTube

Chemistry - ELECTROCHEMICAL SERIES AND ITS APPLICATION:- A list of elements arranged in order on the basis of their standard reduction potential or oxidation potential is called electrochemical series. EXPLAINATION:- Different elements
The standard oxidation potential of Ni|Ni2+ electrode = 0.236 V. If this is combined with a hydrogen electrode in acid solution, at what pH of the solution will the measured emf be