![FTIR spectra of a Ni(Sal)2, b [Cr(en)3]Cl3.3H2O, and c NiCr2O4 (sample 2) | Download Scientific Diagram FTIR spectra of a Ni(Sal)2, b [Cr(en)3]Cl3.3H2O, and c NiCr2O4 (sample 2) | Download Scientific Diagram](https://www.researchgate.net/publication/313686161/figure/fig1/AS:941596942024714@1601505613109/FTIR-spectra-of-a-NiSal2-b-Cren3Cl33H2O-and-c-NiCr2O4-sample-2.gif)
FTIR spectra of a Ni(Sal)2, b [Cr(en)3]Cl3.3H2O, and c NiCr2O4 (sample 2) | Download Scientific Diagram
![Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4] Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4]](https://i.ytimg.com/vi/VpeKIvrO0ZQ/maxresdefault.jpg)
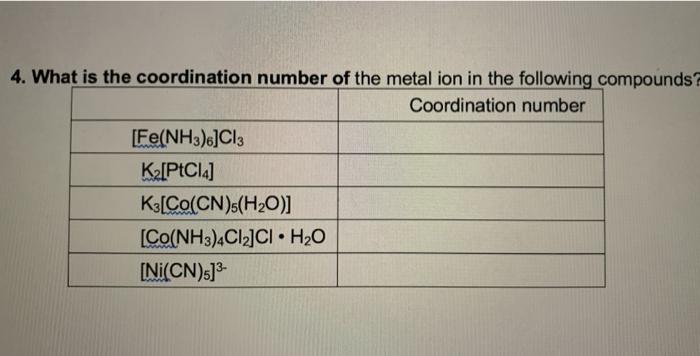
Write IUPAC names of the following coordination compounds.a) [Co(NH3)6]Cl3 b) K3[Fe(CN)6] c) K2[Pd(Cl)4] d) [Ni(Co)4]
Among (i) [Co(NH3)6]Cl3, (ii) [Ni(NH3)6]Cl2, (iii) [Cr(H2O)6]Cl3, (iv) [Fe(H2O)6]Cl2 the complex which is diamagnetic is - Sarthaks eConnect | Largest Online Education Community
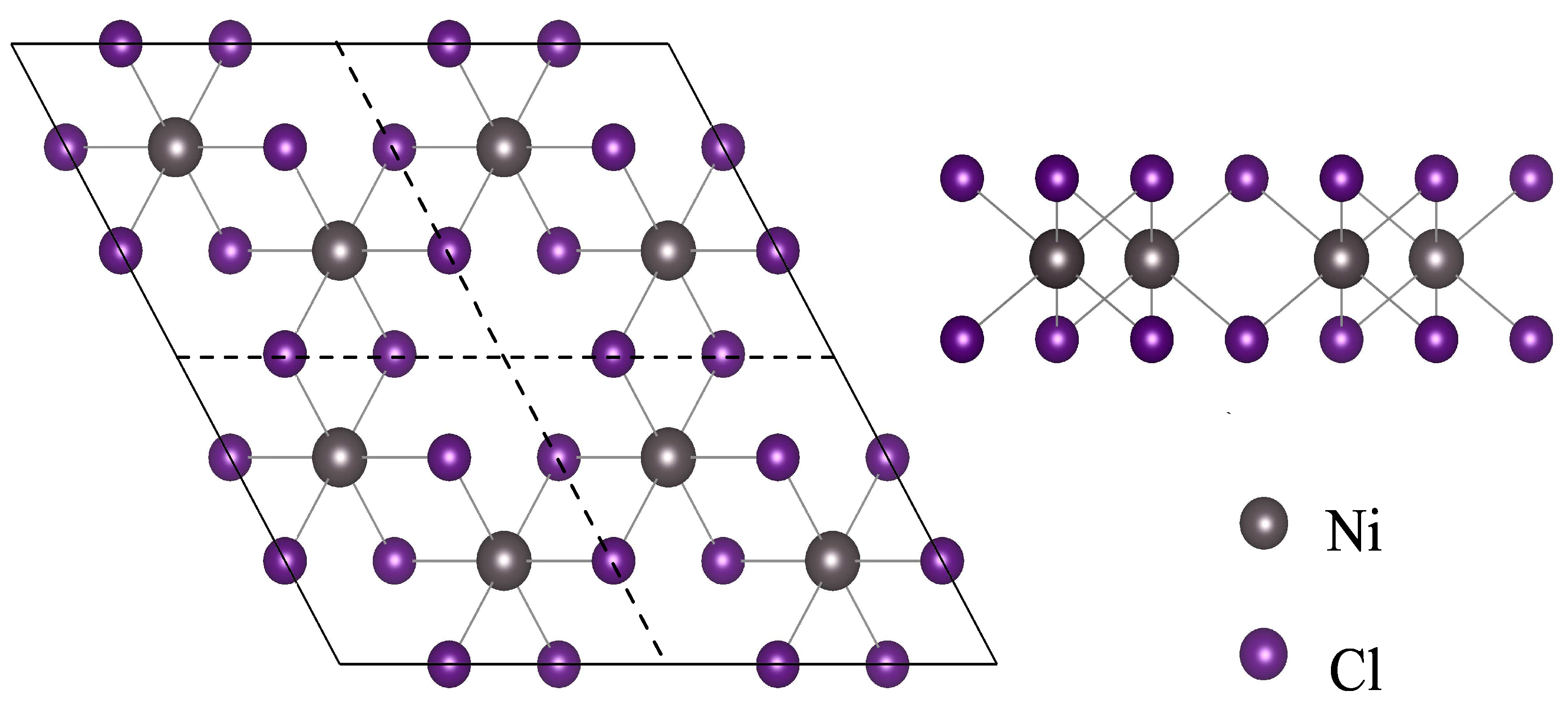
Nanomaterials | Free Full-Text | Thermoelectric Properties of NiCl3 Monolayer: A First-Principles-Based Transport Study

OneClass: NiCl3 is a strong electrolyte. Determine the concentration ofeach of the individual ions in...

SOLVED: How many Ampers (A) are needed to deposit 0.4685 g of Ni metal from an aqueous solution of NiCl3, in 495 seconds?
![Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1280/0*8wwL8Ru43LyLIIL8.png)
Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
![Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number](https://i.pinimg.com/736x/cb/55/b9/cb55b933ee9ec4d59fdf9d226cf3a51a.jpg)
Ni(NH3)6]Cl2 paramagnetic but [Co(NH3)6]Cl3 is diamagnetic - CHEMSOLVE.NET | Electron configuration, Crystal field theory, Coordination number

Ions Atoms that lose electrons (negative) have more positive charge than negative charge so they are positive ions. Atoms that gain electrons (negative) - ppt download
![Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium Why Is [Ni(NH3)6]Cl2 Paramagnetic But [Co(NH3)6]Cl3 Is Diamagnetic ? | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium](https://miro.medium.com/v2/resize:fit:1090/0*rkWRh7JsXBvtOfdK.jpg)
![Solved 5. Name the following compounds. a) [Cr(NH3)6]Cl3 b) | Chegg.com Solved 5. Name the following compounds. a) [Cr(NH3)6]Cl3 b) | Chegg.com](https://media.cheggcdn.com/media/a6d/s918x302/a6df084b-878c-469e-989e-bb7aab4ee9c3/image.png)
![OneClass: What are all the isomers of [NiCl3(H2O)][PF6]? OneClass: What are all the isomers of [NiCl3(H2O)][PF6]?](https://prealliance-textbook-qa.oneclass.com/qa_images/homework_help/question/qa_images/121/12105789.png)
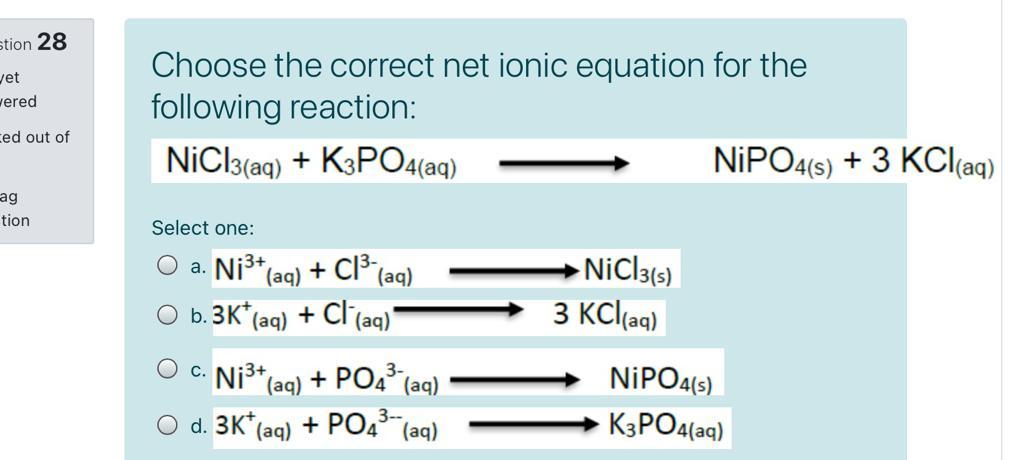

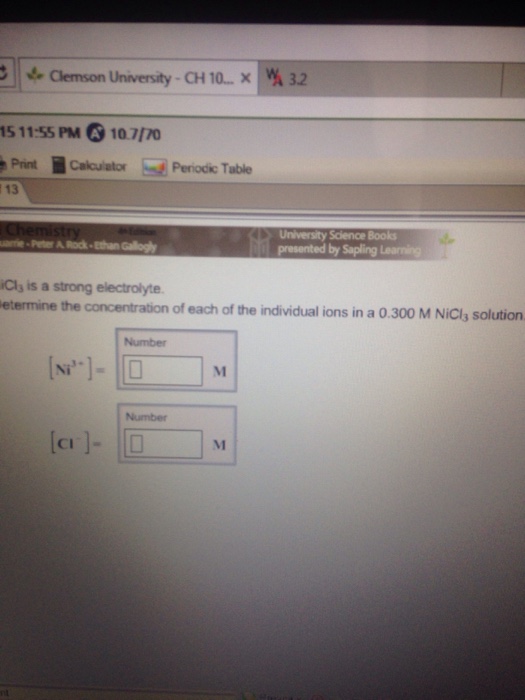


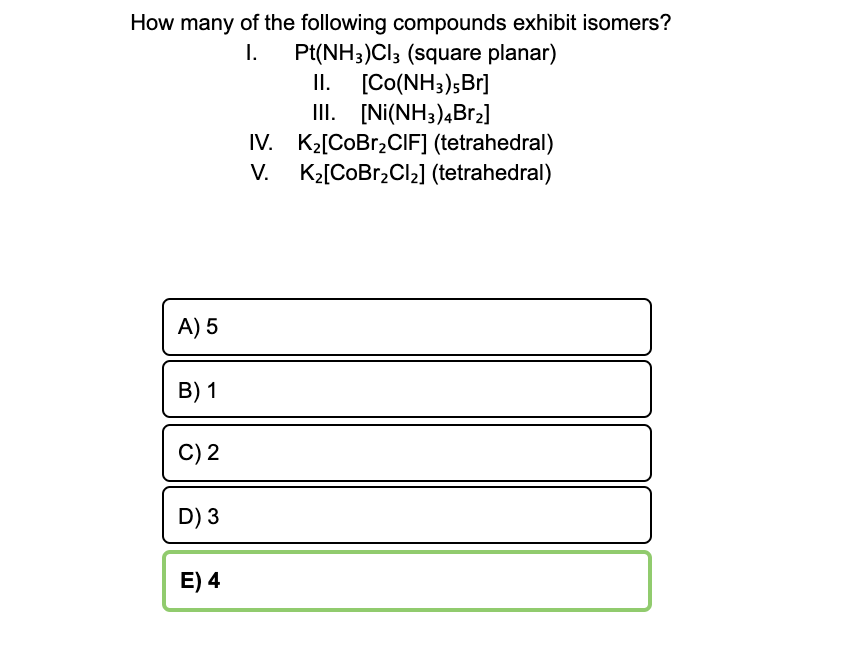

![PDF] NiCl3 Monolayer: Dirac Spin-Gapless Semiconductor and Chern Insulator | Semantic Scholar PDF] NiCl3 Monolayer: Dirac Spin-Gapless Semiconductor and Chern Insulator | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/2c823e3baa5056e082b087e7244c0d89871a55a8/4-Figure1-1.png)
