Photoactive Water-Soluble Vitamin K: A Novel Amphiphilic Photoinduced Antibacterial Agent | ACS Sustainable Chemistry & Engineering
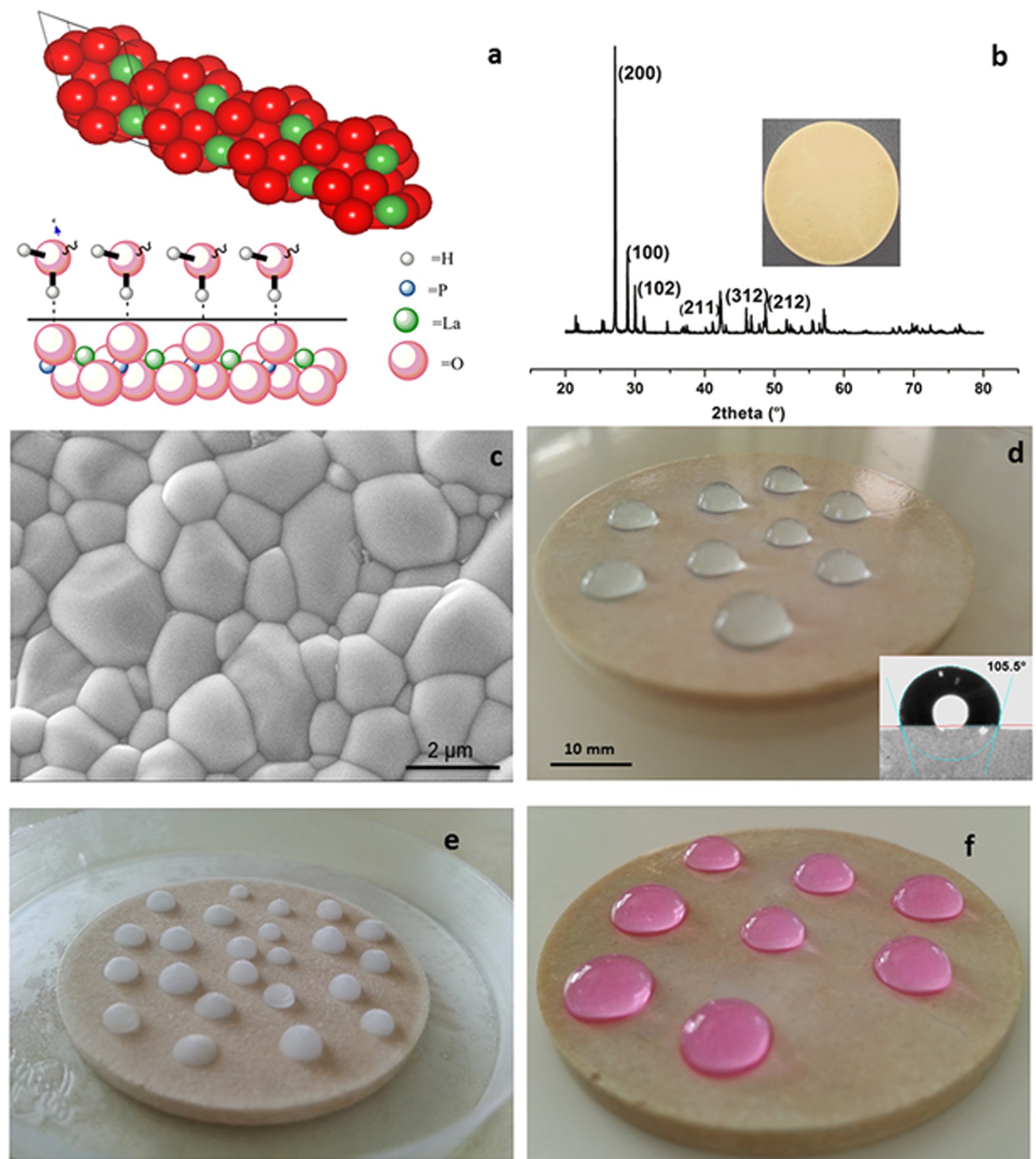
Hydrophobic and Metallophobic Surfaces: Highly Stable Non-wetting Inorganic Surfaces Based on Lanthanum Phosphate Nanorods | Scientific Reports

Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms | Environmental Science & Technology
![Synthesis of the Hydroxide Cluster [Al13(μ3-OH)6(μ-OH)18(H2O)24]15+ from an Aqueous Solution | Inorganic Chemistry Synthesis of the Hydroxide Cluster [Al13(μ3-OH)6(μ-OH)18(H2O)24]15+ from an Aqueous Solution | Inorganic Chemistry](https://pubs.acs.org/cms/10.1021/ic200483q/asset/images/ic200483q.social.jpeg_v03)
Synthesis of the Hydroxide Cluster [Al13(μ3-OH)6(μ-OH)18(H2O)24]15+ from an Aqueous Solution | Inorganic Chemistry

Phosphate sequestration by lanthanum-layered rare earth hydroxides through multiple mechanisms while avoiding the attenuation effect from sediment particles in lake water - ScienceDirect

Lewis Adduct-Dissociating Hydrolysis of Boratrane for Water-Triggered Dehydration of Copolymers with a Hydrophobic Moiety | ACS Macro Letters

Synthetic Receptors with Micromolar Affinity for Chloride in Water - Sudan - Angewandte Chemie International Edition - Wiley Online Library

Ion Hydration and Association in Aqueous Potassium Phosphate Solutions | The Journal of Physical Chemistry B
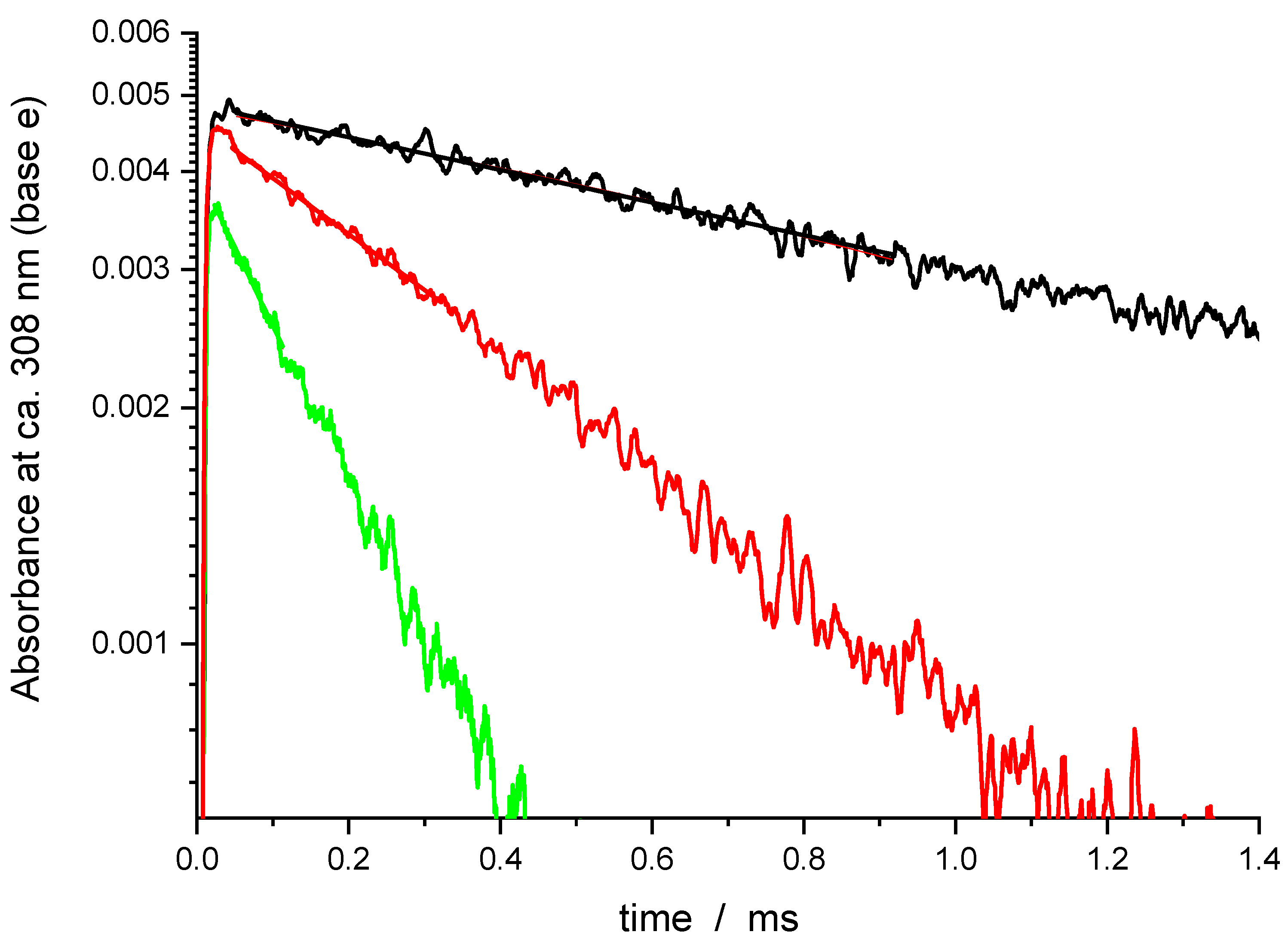
Molecules | Free Full-Text | Kinetics of the Gas-Phase Reaction of Hydroxyl Radicals with Dimethyl Methylphosphonate (DMMP) over an Extended Temperature Range (273–837 K)

Comparative Study of Sodium Phosphate and Sodium Sulfate in Aqueous Solutions at (298.15 to 353.15) K | Journal of Chemical & Engineering Data

Cation Effects on Interfacial Water Organization of Aqueous Chloride Solutions. I. Monovalent Cations: Li+, Na+, K+, and NH4+ | The Journal of Physical Chemistry B
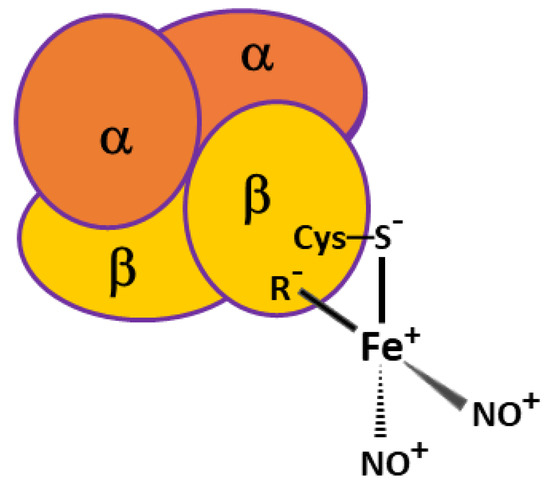
IJMS | Free Full-Text | Protective Effect of Dinitrosyl Iron Complexes Bound with Hemoglobin on Oxidative Modification by Peroxynitrite

pH-Dependent Partitioning of Ionizable Organic Chemicals between the Silicone Polymer Polydimethylsiloxane (PDMS) and Water | ACS Environmental Au

Solid−liquid phase equilibrium for the ternary system (ammonium dihydrogen phosphate + agricultural grade ammonium polyphosphate (degree of polymerization ranged from 1 to 2) + water) at (278.2 and 313.2) K - ScienceDirect

Liquid-liquid equilibrium data for systems formed by PEG (4000 or 6000) or alcohol (1-propanol or 2-propanol) + potassium phosphate + water: Experimental measurements, correlations and thermodynamic modeling - ScienceDirect