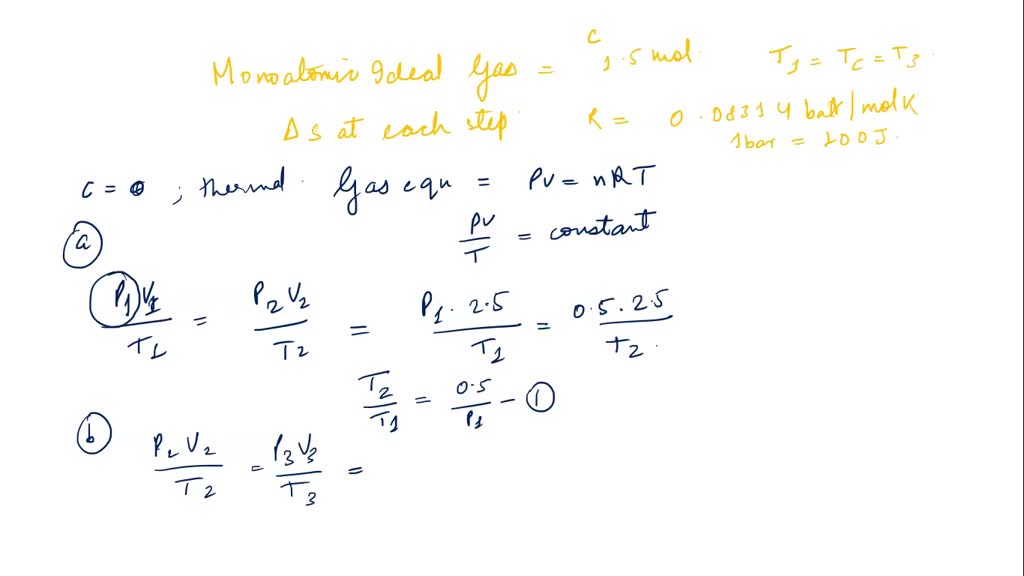
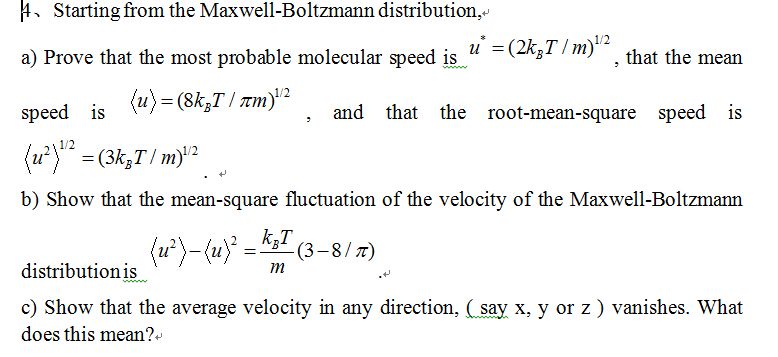
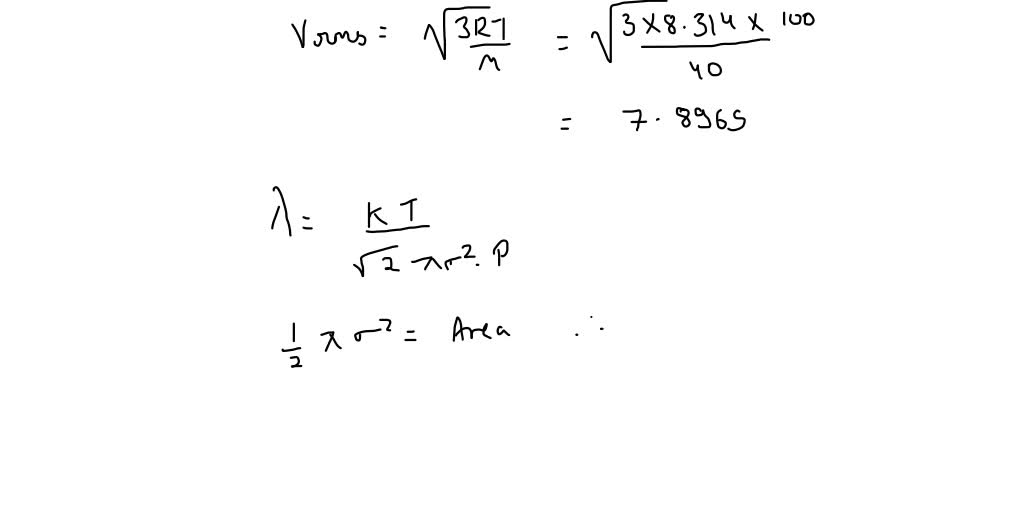Show that the average speed of gas molecule <ν>= √(8kT/πm). - Sarthaks eConnect | Largest Online Education Community

Coefficients of Cauchy dispersion equation of PIMNT single crystals... | Download Scientific Diagram

Ion Solvation Engineering: How to Manipulate the Multiplicity of the Coordination Environment of Multivalent Ions | The Journal of Physical Chemistry Letters

Ion Solvation Engineering: How to Manipulate the Multiplicity of the Coordination Environment of Multivalent Ions | The Journal of Physical Chemistry Letters

SOLVED: The molecular dlameter of CO Is 3.19x 10 " cm: At 600 K and 3 pressure of 200 mm Hg what will the number of molecules colliding per cubic centimeter per

Ion Solvation Engineering: How to Manipulate the Multiplicity of the Coordination Environment of Multivalent Ions | The Journal of Physical Chemistry Letters

SOLVED: Consider the following samples of gas: sample composition pressure temperature 2.1 mol Ar 1.7 atm 282 "C sample composition pressure temperature 1.9 mol Ne 2.3 atm 1.1 mol Ar 1.7 atm

Coefficients of Cauchy dispersion equation of PIMNT single crystals... | Download Scientific Diagram

SOLVED: The molecular dlameter of CO Is 3.19x 10 " cm: At 600 K and 3 pressure of 200 mm Hg what will the number of molecules colliding per cubic centimeter per

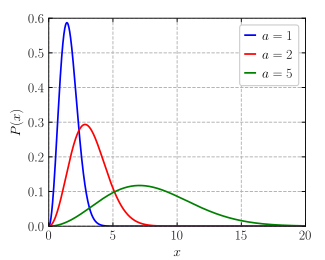
SOLVED: What, according to the Maxwell-Boltzmann distribution, is the proportion of gas molecules having (a) more than, (b) less than the root mean square speed? (c) what are the proportions having speed